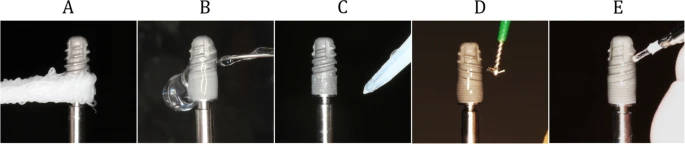
Figure 3. Decontamination methods. a Gauze soaked in saline applied using a sawing motion. b Ultrasonic scaler (SUPRASSON P-MAX, Satelec-Acteon group, Bordeaux, France, power setting: P5, tip: Implant Protect IP3L/R).
Figure 3. Decontamination methods
author: Motohiro Otsuki, Masahiro Wada, Masaya Yamaguchi, Shigetada Kawabata, Yoshinobu Maeda Kazunori Ikebe | publisher: drg. Andreas Tjandra, Sp. Perio, FISID
Serial posts:
- Evaluation of decontamination methods of oral biofilms formed on screw-shaped, rough and machined surface implants: an ex vivo study
- Background : Evaluation of decontamination methods of oral biofilms formed on screw-shaped, rough and machined surface implants
- Materials & methods : Evaluation of decontamination methods on implants (1)
- Materials & methods : Evaluation of decontamination methods on implants (2)
- Materials & methods : Evaluation of decontamination methods on implants (3)
- Results : Evaluation of decontamination methods on implants (3)
- Discussion : Evaluation of decontamination methods on implants (1)
- Discussion : Evaluation of decontamination methods on implants (2)
- Discussion : Evaluation of decontamination methods on implants (3)
- Discussion : Evaluation of decontamination methods on implants (4)
- Discussion : Evaluation of decontamination methods on implants (5)
- Discussion : Evaluation of decontamination methods on implants (6)
- Discussion : Evaluation of decontamination methods on implants (7)
- Discussion : Evaluation of decontamination methods on implants (8)
- Discussion : Evaluation of decontamination methods on implants (9)
- Figure 1. Hard resin splint model carrying 6 implants
- Figure 2. GC Aadva® implant; 3.3-mm diameter, 8-mm length
- Figure 3. Decontamination methods
- Figure 4. SEM analysis of 4 areas. 1 Rough surface—microthread area
- Figure 5. Quantitative analysis of CFU counts on implants
- Figure 6. Comparison of cleansability of each decontamination method
- Table 1 Qualitative evaluation by SEM analysis of micro- and macrothread areas of rough surface implants
- Table 2 Qualitative evaluation by SEM analysis of micro- and macrothread areas of machined surface implants
- Table 3 Quantitative analysis of CFU counts