Operative phase : Combination Syndrome
Operative phase
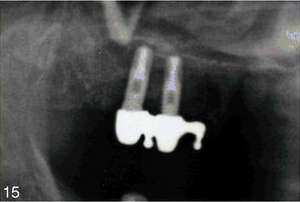
The procedure (surgical stage 1) was started with the administration of two capsules of lidocaine 2% with epinephrine 1:100 000 for the upper right quadrant. The buccal full-thickness flap from the right maxillary tuberosity to the premolar area with the anterior releasing incision was developed. The maxillary sinus was exposed by removing a small amount of anterior maxillary wall bone mesial to the tooth No. 2 with a No. 6 round diamond bur (Osteomed, Addison, Tx). The floor of the maxillary sinus and a Schneiderian membrane were visualized. The membrane was then carefully elevated with sinus curettes (Salvin Dental Specialties, Inc., Charlotte, NC) and tooth No. 2 was extracted atraumatically with a periotome and universal upper forceps. The volume of preserved alveolar bone, in agreement with the preoperative tomographic images, appeared sufficient for the placement of two standard diameter implants in the area No. 2 and 3. Two Lifecore implants (Lifecore Biomedical, Inc., Chaska, Minn) were inserted into the bone with help of the surgical stent: implant No. 2 was placed into the interradicular bone between the anterior and posterior buccal root sockets of extracted tooth No. 2, implant No. 3 was inserted 7 mm anteriorly and parallel to No. 2. Both Lifecore implants were external hex (Restore), 4 mm in diameter, and 11.5 mm in length. About 2 mm of the apical portion of both implants were projecting into the sinus cavity above the sinus floor and below the elevated Schneiderian membrane. The 3 cm3 of cancellous bone grafting material, consisting of autogenous bone that was harvested from the adjacent maxillary tuberosity, was placed in the created subantral pocket around the implants. The buccal flap was then mobilized and closed primarily with 4–0 chromic gut (Henry Schein Inc., Melville, NY). A panoramic X ray after the procedure confirmed good placement of both implants. Patient took amoxicillin, 500 mg (2 g 1 hour before the surgery and 500 mg 3 times daily for 5 days postoperatively) for infection control, and ibuprofen 600 mg as needed for pain. A postoperative period was uneventful.
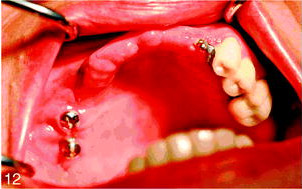
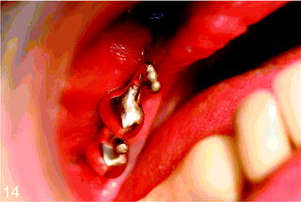
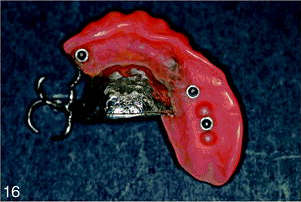
Surgical stage 2 was done 6 months after stage 1. Under local anesthesia, a small buccal flap was developed and both implants were exposed and found to be rigid and fully integrated into the bone. Two temporary healing abutments were placed and the flaps were closed around them (Figures 11 and 12). One month later the patient was referred for the restorative stage.

Serial posts:
- Combination Syndrome: Classification and Case Report
- Introduction: Combination Syndrome
- Case report: combination syndrome
- Diagnosis: Combination Syndrome
- Treatment Plan : Combination Syndrome
- Operative phase : Combination Syndrome
- Restorative stage : Combination Syndrome
- Classification : Combination Syndrome
- Discussion : Combination Syndrome
- Conclusion : Combination Syndrome
- References : Combination Syndrome
- Table Classification of combination syndrome (CS)
- Extended table of classification of Combination Syndrome (CS)