Results: Advanced Platelet-Rich Fibrin
Results
Histochemical studies (H&E, Mason-Goldner, and Giemsa)
S-PRF
In the longitudinal section of the S-PRF clot, produced according to the standard centrifugation protocol (2700 rpm, 12 minutes), a dense fibrin clot was seen with minimal interfibrous space. With the standard histochemical staining methods, cells were observed throughout the clot, albeit decreasing toward the more distal parts of the PRF clot (data not shown).
A-PRF
PRF clots formed with the A-PRF centrifugation protocol (1500 rpm, 14 minutes) showed a looser structure with more interfibrous space, and more cells could be counted in the fibrin-rich clot. Furthermore, the cells were more evenly distributed throughout the clot as compared to S-PRF, and some cells could be found even in the clot's more distal parts. A representative image for cell distribution within A-PRF is given in Figure 2.
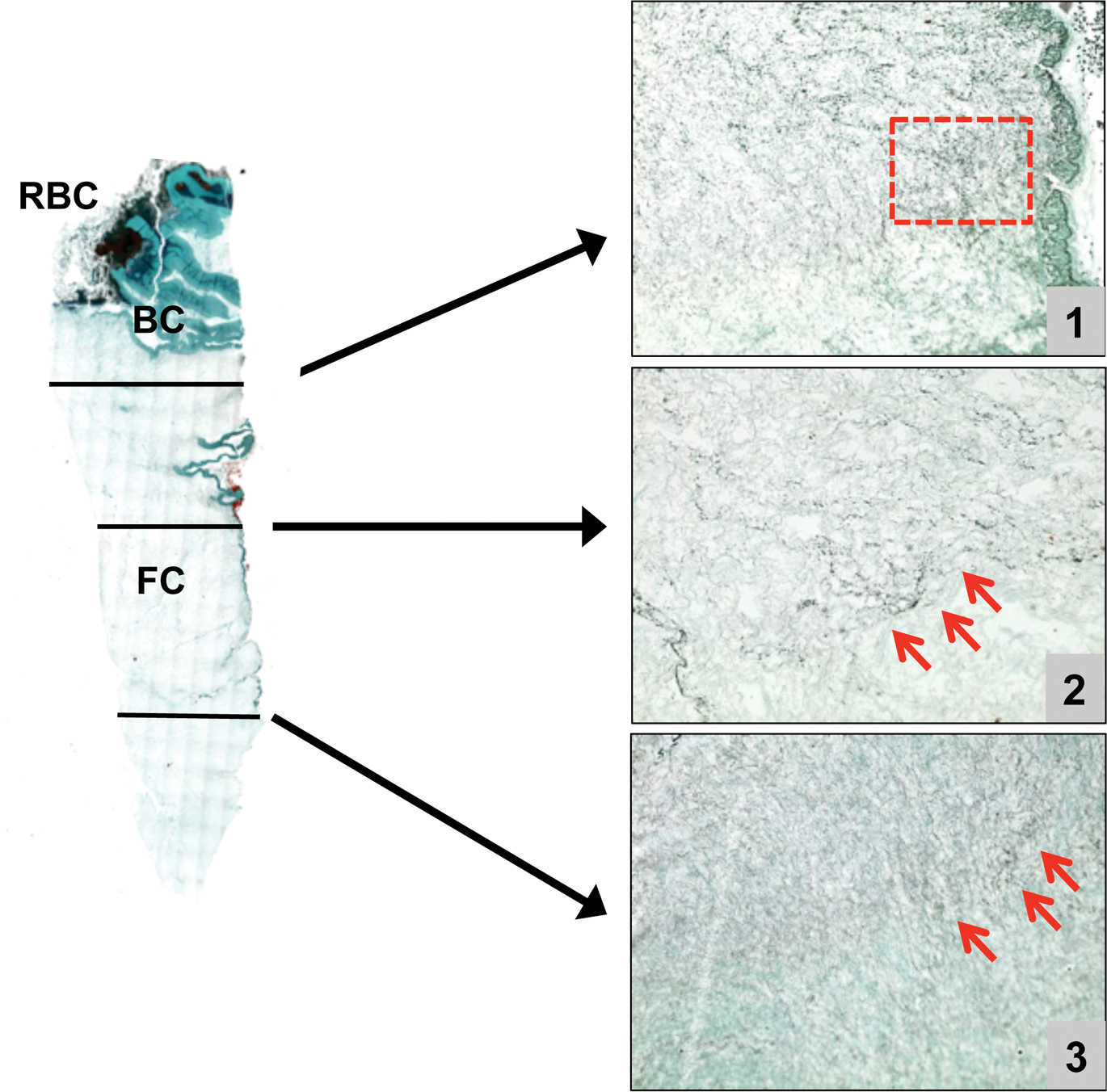
Descriptive immunohistochemical evaluation
S-PRF
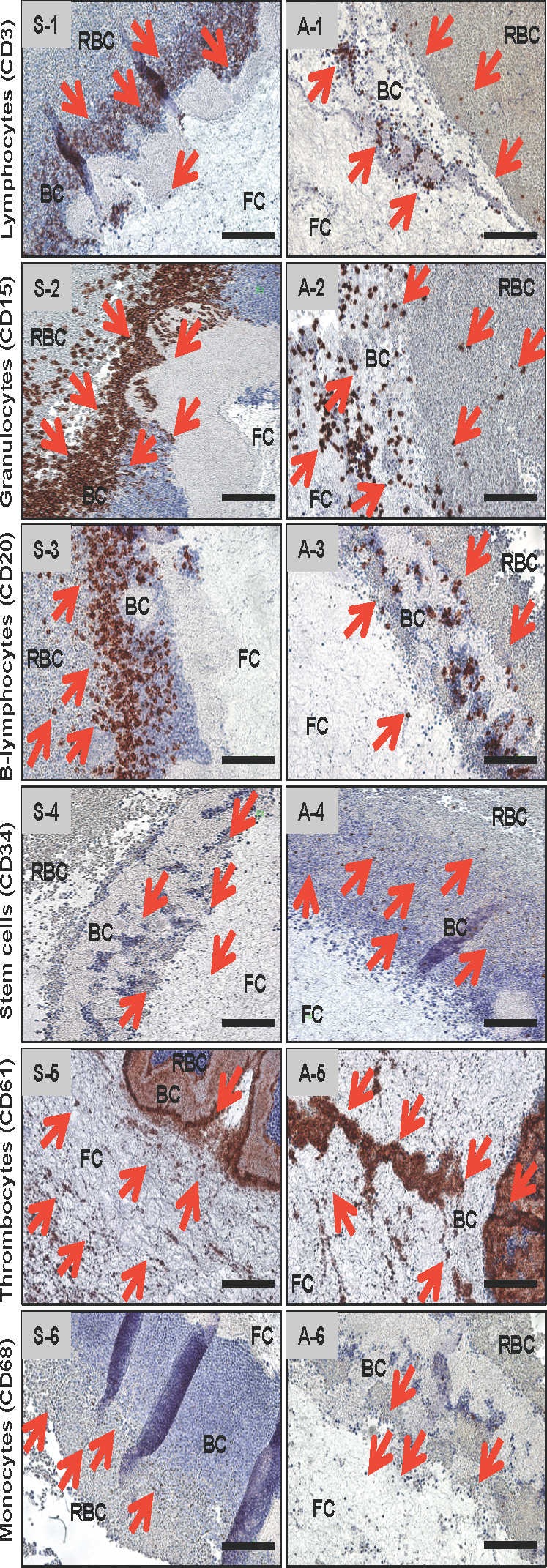
The slides stained for the respective markers were evaluated in terms of specific cell type distribution (Figures 3 and 4). In general, the highest number of positively labeled cells was present in close proximity to the RBC or in the BC. In particular, cells with positive immunolabeling—including T-lymphocytes (CD3-positive cells), B-lymphocytes (CD20-positive cells), stem cells (CD34-positive cells), and monocytes (CD68-positive cells)—were found at the transition zone of the RBC fraction, BC, and proximal parts of the clot. The platelets (CD61-positive cells) were distributed throughout the clot. However, decreased numbers were observed from the proximal (near the BC) to the distal part of the S-PRF clot. Additionally, neutrophilic granulocytes (CD15-positive) showed a tendency to accumulate mainly in the RBC-BC clot interface.
A-PRF
Analogous to the S-PRF, slides were stained immunohistochemically for the markers of interest (Figures 3 and 4). Most of the CD3-, CD20-, CD34-, and CD68-positive cells stained in or near the BC (ie, the very proximal part of the fibrin clot); however, the BC was more extensive in comparison to S-PRF. Additionally, the neutrophilic granulocytes (ie, CD15-positive cells) were distributed more widely toward the distal (ie, away from the BC) part of the fibrin-clot. Approximately two-thirds of the clot was seeded with neutrophilic granulocytes/CD15-positive cells, with only the last third (distal part of the fibrin clot) spared. As has been observed in the S-PRF group, platelets (ie, CD61-positive) were found throughout the entire clot. When compared to the S-PRF group, the amount of CD61-positive cells did not decrease to the same extent in the periphery.
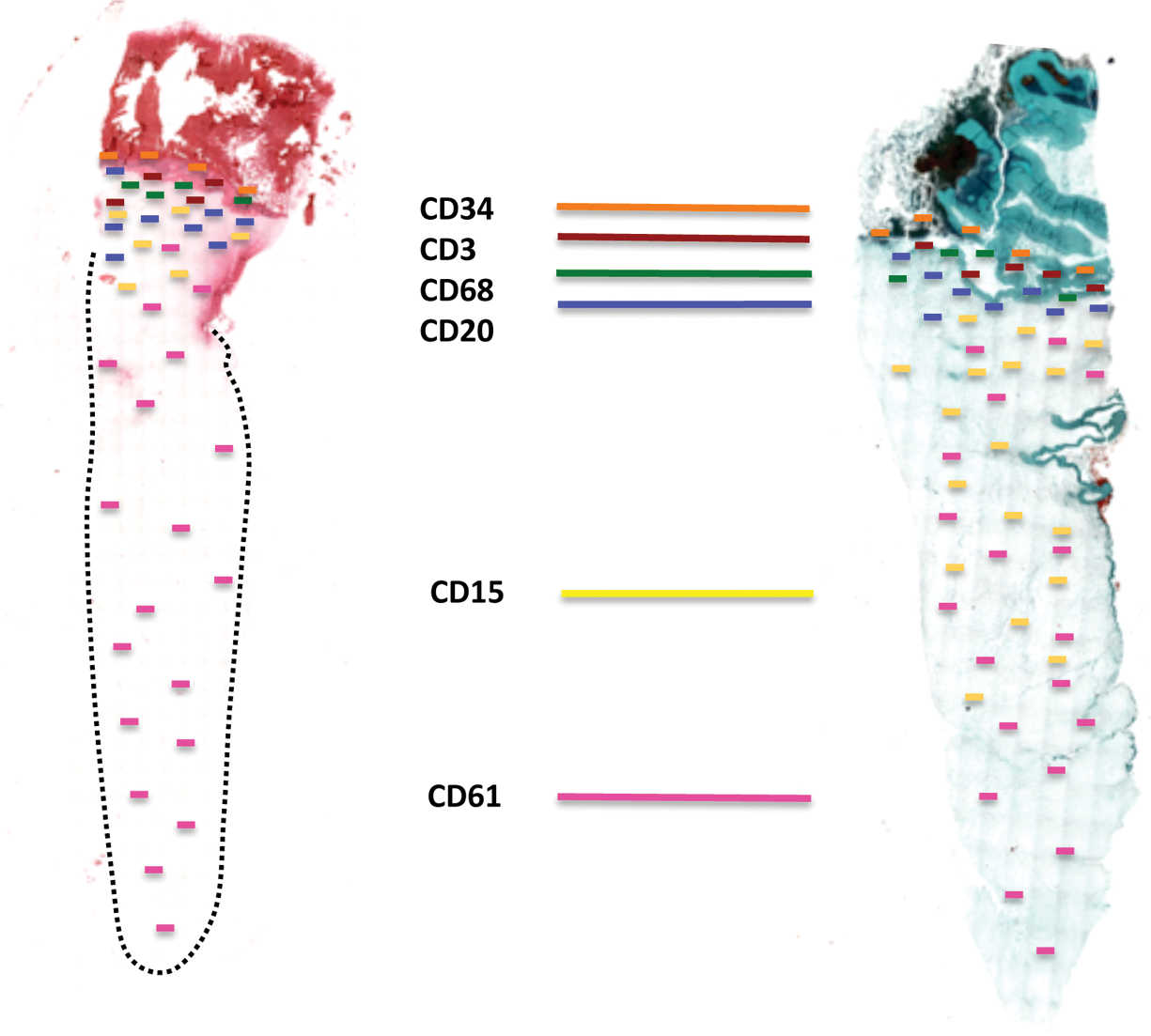
Quantitative histomorphometric analysis of cell penetration
The histomorphometrical analysis of the total scans permitted an evaluation and comparison of the respective cell distribution in the PRF clots. The total length of each clot was measured and a mean of ± SEM was calculated. The distribution/allocation of each cell type was evaluated in the corresponding total scan of the immunohistochemical staining. The analyses revealed that platelets were the only ones found in each area of the clot up to 87 ± 13% in the S-PRF group and up to 84 ± 16% in the A-PRF group (Figure 5). Furthermore, the results showed that T-lymphocytes (S-PRF: 12 ± 5%, A-PRF: 17 ± 9%), B-lymphocytes (S-PRF: 14 ± 7%, A-PRF: 12 ± 9%), CD34-positive stem cells (S-PRF: 17 ± 6%, A-PRF: 21 ± 11%), and monocytes (S-PRF: 19 ± 9%, A-PRF: 22 ± 8%) were not found beyond a certain point of maximally 30% of the total clot length, as they are distributed in or near the BC generated by the centrifugation process (Figure 5). Statistical analysis revealed no statistically significant differences between the values of the two groups concerning the allocation of these cell types (Figure 5). It is a remarkable observation that the changes in parameters in PRF centrifugation protocols result in an increased cell distribution of neutrophilic granulocytes up to 68 ± 24% of the scaffold within the A-PRF group, while this cell type was located up to 25 ± 12% of the clot length in the S-PRF group (Figure 5). In this case, statistical analysis showed a highly significant difference regarding the “distribution” depth of this cell type between both study groups (**P < .01) (Figure 5).
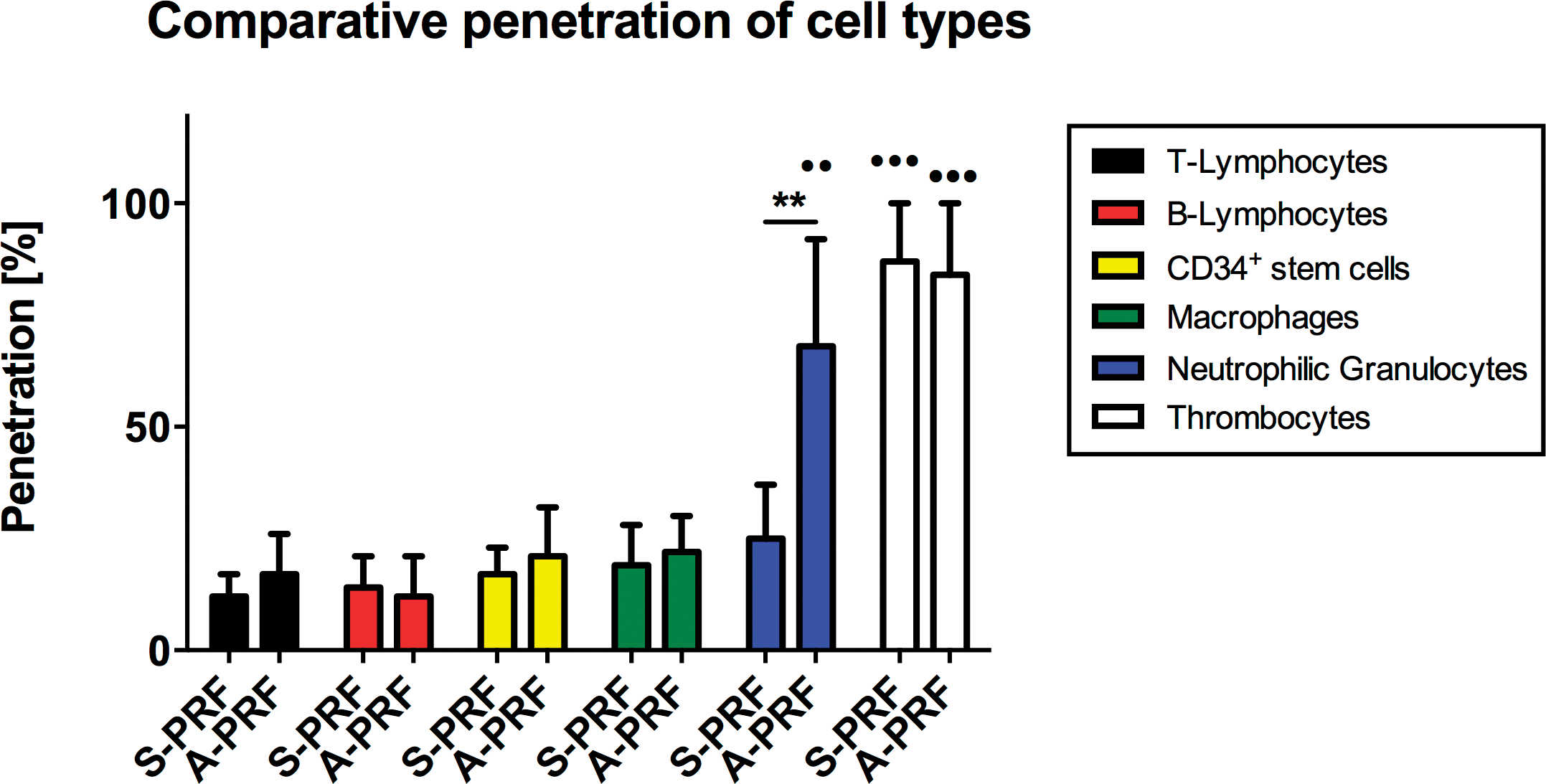
Additionally, no significant differences in the distribution depths between the platelets, T- lymphocytes, B-lymphocytes, stem cells, and monocytes were found when comparing the two centrifugation steps (Figure 5). Furthermore, the analyses showed that the distribution depth of the neutrophilic granulocytes in the A-PRF group was highly increased compared to the before-mentioned cell types (**P < .01), while no interindividual differences were found in the S-PRF group between these cell types (Figure 5). Finally, the distribution depths of the platelets were significantly higher compared to the values of all other cell types in both study groups (***P < .001) (Figure 5).